This Dr. Axe content is medically reviewed or fact checked to ensure factually accurate information.
With strict editorial sourcing guidelines, we only link to academic research institutions, reputable media sites and, when research is available, medically peer-reviewed studies. Note that the numbers in parentheses (1, 2, etc.) are clickable links to these studies.
The information in our articles is NOT intended to replace a one-on-one relationship with a qualified health care professional and is not intended as medical advice.
This article is based on scientific evidence, written by experts and fact checked by our trained editorial staff. Note that the numbers in parentheses (1, 2, etc.) are clickable links to medically peer-reviewed studies.
Our team includes licensed nutritionists and dietitians, certified health education specialists, as well as certified strength and conditioning specialists, personal trainers and corrective exercise specialists. Our team aims to be not only thorough with its research, but also objective and unbiased.
The information in our articles is NOT intended to replace a one-on-one relationship with a qualified health care professional and is not intended as medical advice.
Breast Implant Illness + 6 Other Breast Implant Dangers
January 29, 2018

The quest for perfection has never been higher. Reality TV, advertisements and the media constantly promote a “perfect” body. The result is more and more women (and men) are turning to plastic surgery. In fact, in 2015 over 1.7 million cosmetic surgical procedures were performed in the United States. (1)
- Breast Augmentation: 279,143
- Liposuction 222,051
- Nose Reshaping 217,979
- Eyelid Surgery 203,934
- Tummy Tucks 127,967
When considering any type of elective surgery, it is imperative that you understand the risks during surgery, potential surgical complications, and any potential for ongoing adverse effects caused by cosmetic procedures.
For individuals considering breast augmentation surgery, take note of the following statement from the FDA:
“Breast implants are not lifetime devices; the longer you have your implants, the more likely it will be for you to have them removed.” (2)
In breast augmentation surgery, implants are inserted under the skin to provide added fullness and to enhance the shape of the breast. While typically considered a safe procedure with minimal risk, millions of women worldwide have developed symptoms after implantation in the 50 years they have been on the market.
These symptoms have been coined “breast implant illness.” From minor irritations to greater health challenges, research supports that in some individuals, both saline-filled and silicone-filled breast implants can cause significant adverse health effects, leading us to question if breast implants are safe. In addition, implants have been found to increase the risk of certain types of cancer. (3)
As of May 2019, the FDA clearly states that there is a risk of women with textured breast implants developing a type of cancer known as anaplastic large-cell lymphoma. Even though the risk of cancer and illness exists, the FDA is allowing continued availability of these implants in the United States, but will take steps for “more transparent medical device reports” to increase public awareness of the possible adverse effects of breast implants. Meanwhile, these implants have been taking off the market in 38 other countries including France and Canada.
Types of Breast Implants
- Saline-Filled Breast Implants: Silicone pockets filled with sterile salt water approved for augmentation in women 18 years or older. Structured saline breast implants have an additional inner structure that lends a more natural feel than the standard saline-filled implants.
- Silicone Gel-Filled Breast Implants: Silicone shells filled with silicone typically feel more like real breasts. However, they pose more of a risk if they leak. (4) Silicone breast implants are FDA-approved for breast augmentation procedures in women 22 or older.
- Textured Breast Implants: Textured breast implants have a rough/bumpy surface and research has shown that the “texturing process may lead to residual surface debris that may be shed from the implant to the patient.”
- NOTE: With silicone gel-filled implants, it is highly recommended that an MRI scan is conducted 3 years after implant, and every 2 years after that to check for a silent rupture. If the implants rupture, they will need to be removed. Be advised that insurance may or may not cover the costs of the MRIs, nor the removal in the event of a rupture.
Manufacturers continue to innovate breast implant design. Now gummy bear breast implants, round breast implants, smooth and textured breast implants are available on the market. (5)
A Brief History of Breast Augmentation
The fascination with the female breast has existed since the beginning of time. And, for the last 120 years, physicians across the globe have been testing ways to enhance the female form. The first record of breast augmentation surgery dates to 1895 when Dr. Vincenz Czerny tested injecting paraffin into the breast that sadly resulted in fistulas, tissue necrosis and granulomas.
Over the next several decades more physicians experimented, often with disastrous results. They implanted glass balls, rubber, wool, foam sponges, ox cartilage and even ivory to create the ideal breast. During the mid-20th century, physicians tested animal fatty acids, olive oil, putty, silicone oil and even snake venom. But nothing provided the look, feel or safety desired. (6)
Then, in the early 1960s, Dow Corning Corporation, along with Thomas Cronin and Frank Gerow, developed the first silicone breast prosthesis. This resulted in the first augmentation surgery in 1962. For the next 30 years, the FDA did not require companies to prove implants were safe. Hundreds of thousands of women in the United States were implanted with silicone breast implants. The reporting of complications, including capsule contractures, necrosis, seromas, ruptures and autoimmune-related symptoms, escalated through the 1980s.
Finally, in 1992 the FDA requested a “voluntary moratorium on the implantation of silicone-filled implants because of the lack of scientific and clinical data supporting their safety.” Many of the symptoms and adverse effects reported to the FDA and other governing bodies around the globe included scleroderma, fibromyalgia and chronic fatigue syndrome. (7) After the FDA’s request, saline-filled implants took over the market.
As women continued to experience adverse effects of silicone-filled breast implants in the mid-1990s, well-known attorney Ed Blizzard, known for taking on pharmaceutical companies, served as counsel for nearly 200,000 women worldwide who were injured or made ill by silicone breast implants produced by Dow Corning. As one of the chief negotiators, he got $3.2 billion for his clients. (8). After this victory, more and more women across the country stepped forward and began legal proceedings against the makers of breast implants.
Dow Corning was not the only manufacturer of breast implants sued during this time; 3M paid $325 million to compensate women who received their silicone breast implants and Union Carbide Corporation agreed to pay $138 million. Bristol-Myers Squibb, Baxter International, and Inamed Inc. also contributed to a settlement agreement. This settlement granted women payments of $200,000 to $2 million for the diagnosis, treatment and removal of leaking silicone breast implants due to the silicone migrating, which causes life-threatening autoimmune disorders like lupus. (9)
After being off-market for a little more than a decade, the FDA approved silicone gel-filled breast implants for augmentation in November 2006, with directives that manufacturers are required to conduct post-operative studies to “further characterize the safety and effectiveness of their silicone gel-filled breast implants and to answer scientific questions that the premarket clinical trials were not designed to answer.” (10)
Early in 2011, the FDA issued a Safety Communication on anaplastic large cell lymphoma (ALCL) in women with breast implants because research indicated that there is an increased risk of developing this rare disease in the scar capsule adjacent to the implant.
Anaplastic large-cell lymphoma is a type of non-Hodgkin lymphoma that is linked to exposure to certain chemicals, immune system deficiency, certain infections and several autoimmune diseases. According to the American Cancer Society, rheumatoid arthritis, systemic lupus erythematosus, Sjogren disease, celiac sprue and other diseases have been linked with an increased rate of non-Hodgkin lymphoma. (11)
What Is Breast Implant Illness?
Breast implant illness is the name given to the variety of symptoms and illnesses reported by women after implantation. Often, allergy-like symptoms including fatigue, muscle weakness, aches and pains, and brain fog begin shortly after breast augmentation surgery. While silicone is used for a variety of medical devices, including pacemakers and cochlear implants, research is now pointing to symptoms appearing after exposure to silicone. (12)
In fact, the 2008 study “The Association Between Silicone Implants and Both Antibodies and Autoimmune Diseases” stated that women with silicone breast implants had a higher IgE serum level than women without silicone breast implants. (13) IgE levels are high when the body’s immune system responds to a perceived threat, releasing additional immunoglobulin E. Elevated concentrations are found in various diseases including: primary immunodeficiencies, infections, inflammatory diseases, and malignancies. (14)
Since 2008, women with breast implants diagnosed with anaplastic large-cell lymphoma in the breast (otherwise known as breast-ALCL) has increased, according to a 2018 study. The study utilized a Dutch pathology registry that held clinical data between 1990 and 2016 to identify all patients diagnosed with primary non-Hodgkin lymphoma in the breast and whether or not they had breast implants. Among 43 identified patients with breast-ALCL, 32 of the women had ipsilateral breast implants, compared with one among 146 women with alternate primary breast lymphomas. Prevalence of breast implants in women aged 20 to 70 years old was 3.3 percent; cumulative risks of breast-ALCL in women with implants were 29 per million at 50 years old and 82 million at 70 years old. The study concludes there is an associated risk of breast-ALCL with breast implants, however the risk still remains minute. (15)
Other symptoms of breast implant illness include depression, panic attacks, short-term memory loss, hair loss and changes in skin.
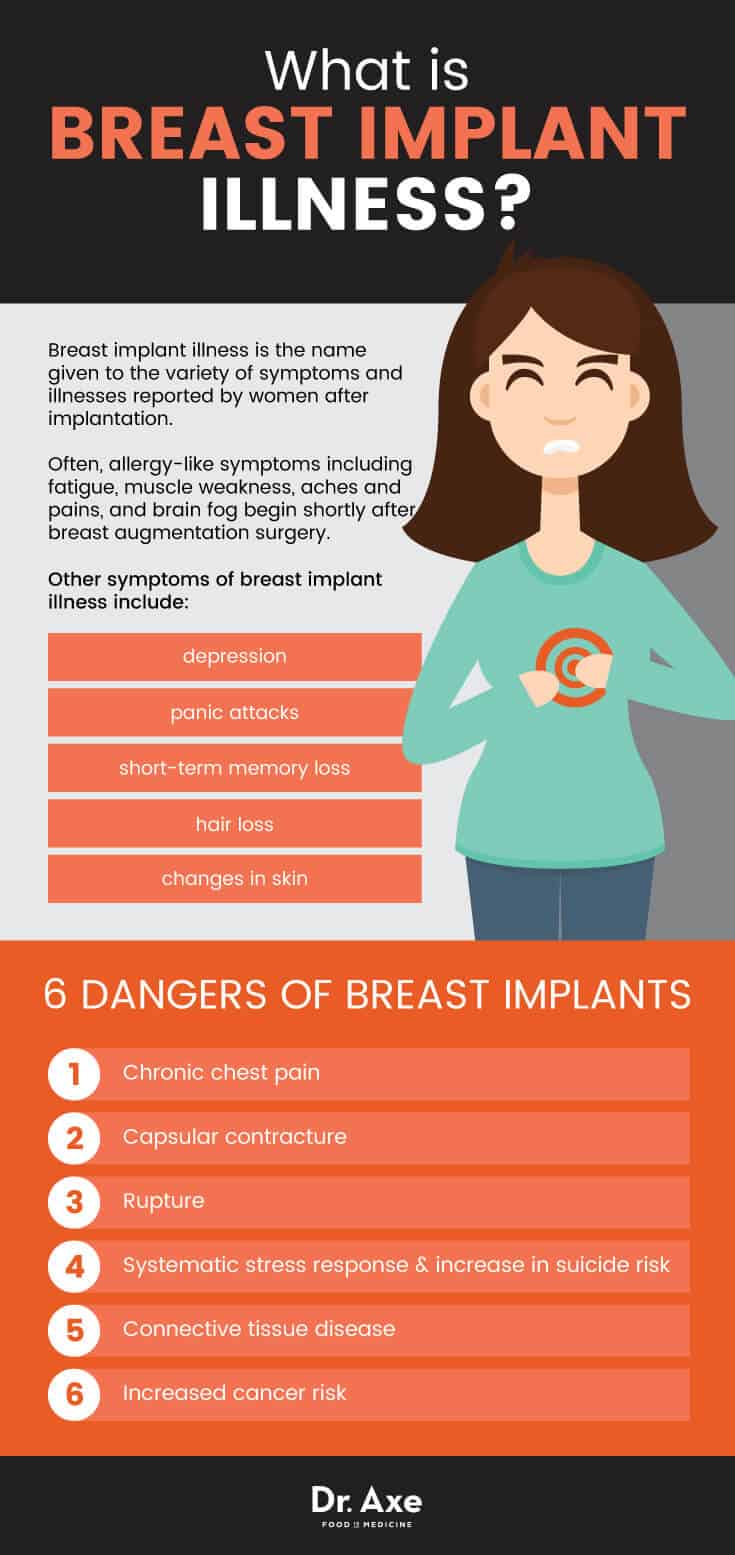
Breast Implant Illness: 6 Dangers of Breast Implants
- Chronic Chest Pain. A small study conducted at Baylor College of Medicine found silicone breast implants may cause an atypical chest pain syndrome, similar to heart attacks. Chest pain was considered severe and at the end of the study, all 11 patients had implants removed, with 5 ruptures noted and an additional 5 patients with free silicone in surrounding tissue, whether the implant was ruptured or not (16), signaling silicone leakage or weeping.
- Capsular Contracture. After implantation of either saline-filled or silicone gel-filled implants, scar tissue known as the “capsule” forms around the implant. In some cases, this scar tissue may tighten and squeeze the implant causing breasts to become uncomfortably firm, visibly distorted, or even painful and hard. Additional surgery may be required to correct this condition and may include the removal of the capsule tissue and removal of the implant. The FDA warns this may happen again after surgical correction. (17)
- Rupture rates, as high as 40% for some models of breast implants (that have now been recalled) are reported with researchers finding that a minimum of 15% of “modern implants” can be expected to rupture between the third and 10th year after implantation. (18)
When a saline-filled breast implant ruptures, the salt water leaks out and the body absorbs it. There have been circumstances in the last few years where mold and bacteria have grown inside saline implants causing adverse side effects. (19)
When a silicone breast implant ruptures, the first signs are breast pain and a change in the shape of the breast. If you suspect your implant has ruptured, speak with your doctor immediately about an MRI scan, especially if you are experiencing any signs or symptoms related to silicone poisoning or toxicity.
Also, when a silicone breast implant ruptures, the immune system attempts to separate the silicone from the body, creating a “siliconoma.” Researchers have recently found that siliconomas can travel throughout the body to reach the extremities. (20)
In the study “Pathology of Silicone Leakage from Breast Implants” conducted by the Department of Pathology at Vrije Universiteit, Amsterdam, researchers outlined that silicone leakage and “gel bleed” is associated with histiocytic necrotizing lymphadenitis and autoimmune and connective tissue diseases. The conclusion drawn was that side effects may be significant enough to lead to further discussion about removing the implants from the market. (21)
- Researchers have found that breast augmentation surgery can stimulate a systematic stress response and increase a pre-existing risk of suicide in women. Researchers found that there is a two- to threefold increased suicide risk among individuals who have had breast augmentation surgery and that further examination and research is necessary. (22)
And, the National Cancer Institute seems to agree stating, “The excess risk of suicide among the implant patients remains of concern.” (23)
- Connective Tissue Disease. In 2001, the Journal of Rheumatology published research conducted by the Office of Surveillance and Biometrics, Center for Devices and Radiological Health of the FDA. Researchers found that women with silicone gel-filled breast implants that had ruptured were significantly more likely to have fibromyalgia, polymyositis, Hashimoto’s thyroiditis, pulmonary fibrosis, eosinophilic fasciitis, and polymyalgia than others in the study.(24)
The study itself stated “If this association persists in other studies, women with silicone gel breast implants should be informed of the potential risk of developing fibromyalgia if their breast implants rupture and the silicone gel escapes the fibrous scar capsule.” However, this significant finding is not included in the FDA’s “Risks of Breast Implants” highlighted below.
The article, “Autoimmune/Inflammatory Syndrome Induced by Adjuvant (ASIA) Evolution After Silicone Implants. Who is at risk?,” published in Clinical Rheumatology, recommends that individuals who have previously diagnosed autoimmune diseases or a genetic preponderance for hyperactive immune system should not be considered candidates for silicone gel-filled breast implants. (25)
- Increased Cancer Risk. In 2001 the National Cancer Institute found that women with breast implants have significantly elevated instances of cancers of the stomach, vulva, brain and leukemia. (26)
In addition, the FDA, like many health organizations, has stated that women with silicone breast implants do have a higher risk for developing the rare anaplastic large cell lymphoma. (27) This type of cancer can be particularly insidious because as of 2015 only about 30 percent of plastic surgeons were discussing risks of this cancer with patients. For some reason that doctors and researchers don’t yet understand, the risk of this rare lymphoma is higher with textured implants rather than smooth implants. If it’s diagnosed early enough, it’s usually treatable and not often fatal. However, as of March 2017 the FDA had received reports of nine deaths as a result of breast implant-induced anaplastic large cell lymphoma. (28)
Researchers at the Zabludowicz Center for Autoimmune Diseases in Israel found that breast implants actually cause a chronic stimulation of the immune system against the prosthetic material. The study recommends that patients who experience an inflammatory response to silicone should be monitored carefully as “serious health effects can follow.”(29)
In addition to the 6 breast implants dangers outlined above, the FDA publishes “The Risks of Breast Implants,” which are: (30)
- Additional Surgeries. Breast implants are not considered lifetime devices. Patients should expect to have surgeries for replacement every 10-15 years.
- Asymmetry. Breasts may not be symmetrical after implantation.
- Breastfeeding. Breastfeeding may or may not be affected by implants. Another consideration is that it is possible that a small amount of silicone may pass through breast implants’ silicone shell into breast milk during breastfeeding. The FDA states that there are no established methods for accurately detecting silicone levels in breast milk. (31)
- Breast Pain. Ongoing pain in the nipple or breast.
- Breast Tissue Atrophy. Thinning and shrinking of the breast tissue and skin.
- Calcification/Calcium Deposits. Hard lumps around the implant that can be mistaken for cancer during a mammography.
- Chest Wall Deformity. Rib cage and chest wall can appear deformed.
- Deflation in Saline Implants. Leakage of saline caused by valve leak, tear or rupture of the silicone shell.
- Delayed Wound Healing. Incision site fails to heal normally.
- Extrusion. The skin breaks down, and the implant appears through the skin.
- Hematomas. Blood collects near the surgical site resulting in swelling, bruising and pain. Large hematomas may require surgical draining.
- Iatrogenic Injury/Damage. Damage to breast tissue or implant as a result of the implant surgery.
- Infection, including Toxic Shock Syndrome. Caused by wounds contaminated with bacteria or fungi. If antibiotics fail, the implant may need to be removed.
- Inflammation/Irritation. Redness, swelling, pain and irritation caused by the body as a result of injury or infection.
- Lymphedema or Lymphadenopathy. Swollen or enlarged lymph nodes.
- Malposition/Displacement. The implant may not be in the correct position after surgery. Shifting can occur due to gravity, trauma or capsular contracture.
- Necrosis. Dead skin or tissue around the breast caused by infection, steroids, smoking, chemotherapy/radiation and excessive heat or cold therapy.
- Nipple/Breast Changes. Increase or decrease in the feeling and sensitivity of the nipple and breast. May affect sexual response or breastfeeding.
- Palpability. The implant is felt through the skin.
- Ptosis. Breast sagging due to aging, pregnancy, or weight loss.
- Redness/Bruising. Bleeding during surgery can cause the skin to change color; it is likely temporary.
- Seroma. Fluid may collect around the implant causing swelling, pain and bruising. The body may absorb small seromas; however, larger ones will require surgical draining.
- Skin Rash. Rash on or around the breast.
- Unsatisfactory Style/Size. The patient is unsatisfied with the overall look.
- Visibility. The implant can be seen through the skin.
- Wrinkling/Rippling. Wrinkling of the implant that can be seen or felt through the skin.
Breast Implant Illness: Consider “Explant” Surgery
Dr. Edward Melmed, a leader in plastic surgery, implanted thousands of women over the years. In 1992 he started taking breast implants out instead of putting them in. (32) “Like most plastic surgeons, I didn’t think there was anything wrong with implants. We were always told that implants would last forever. We know that is not true anymore.”
Now, women from around the globe suffering from breast implant illness and other symptoms seek out Dr. Melmed, and other plastic surgeons that are willing to remove breast implants and not replace them. According to the American Society of Plastic Surgeons, 28,467 implant removals were completed in 2016. (33)
Research supports explantation for individuals experiencing adverse effects and symptoms. Recently, researchers from the Department of Plastic and Reconstructive Surgery, VU University Medical Center in Amsterdam, stated that explantation of the implants may reduce the symptoms, including fatigue, joint and muscle pain, morning stiffness, night sweats, cognitive and dermatological complaints. (34)
If you are experiencing any of the symptoms mentioned above, or have been diagnosed with an autoimmune-related disease, having your implants removed may provide the relief you’ve been seeking.
Breast Implant Illness: A Special Note for Women with Breast Cancer Considering Breast Implants After a Mastectomy
While implants are the typical choice for breast reconstruction after a mastectomy, I encourage women facing this decision to evaluate all options fully. My concern is that introducing a foreign body to your body while you are still in the process of healing from breast cancer may cause additional side effects and delay healing.
In addition to silicone or saline breast implants, there are procedures that use your own tissue for the reconstruction; they are commonly called “tissue flaps.” The TRAM flaps and DIEP flaps both use tissue from the tummy. The GAP flaps use tissue from the glutes and TUG flaps use tissue from the inner thighs. (35)
While these surgeries do require multiple surgical sites and a longer recovery, it is important to note that you aren’t introducing a foreign body into your system that has caused an autoimmune response in some women.
Although not for everyone, some women who have had mastectomies are opting for tattoos instead of reconstruction to cover the scars left behind. P-INK, a nonprofit organization, brings together breast cancer survivors and tattoo artists who create true works of art across scar tissue canvas.
Final Thoughts Breast Implant Illness
Are breast implants safe? The controversy surrounding breast implants, their safety, and related complications and dangers has been debated for decades. According to the American Society of Plastic Surgeons, in 2016, 84% of total breast implant surgeries used silicone implants, and nearly 300,000 breast augmentation surgeries were completed.
The risks for developing certain types of cancer, autoimmune diseases, suicide, chronic chest pain, capsular contracture and rupture seem too great. I have to believe that if the public understood these elevated risks, more would choose not to have breast augmentation surgery.
I keep coming back to the FDA’s statement, “Breast implants are not lifetime devices; the longer you have your implants, the more likely it will be for you to have them removed.”
In addition, breast implants require special breast cancer screenings, MRIs to detect silent ruptures, and new surgeries to remove and replace implants every 10-15 years. Also, remember, as breast implant surgery is an elective surgery, your insurance company is not obligated to pay for the special breast cancer screenings, MRIs or in the event of a rupture, to replace implants.
The risks, in my opinion, do outweigh the benefits.